Borophene is a two-dimensional monoatomic material first synthesized in 2015. Its properties are still being studied, but it is already called the “killer of graphene”, for the production of which fantastic amounts of money have recently been allocated.
EU officials have already admitted that the allocation of €1 billion for the production of graphene was a hasty decision. But it can hardly be called a mistake, because it was the excitement around that material that led to the discovery of its superior analogue.
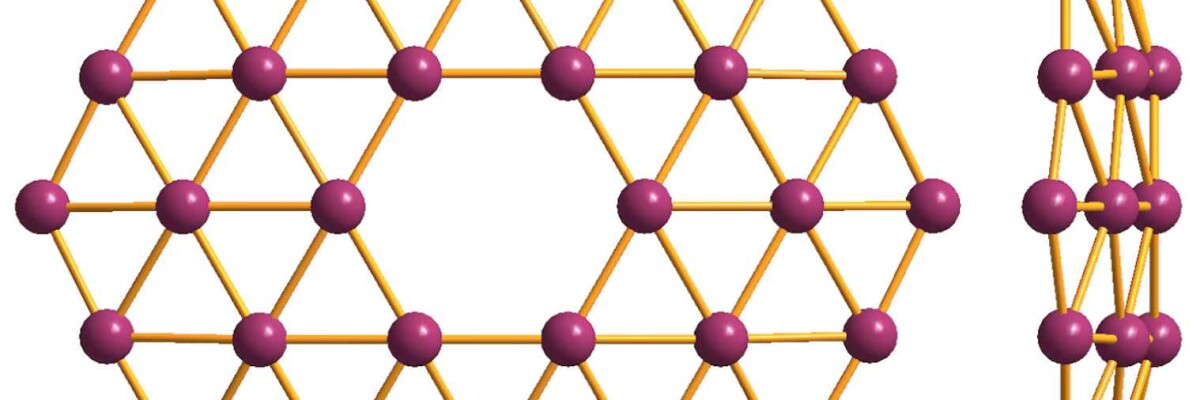
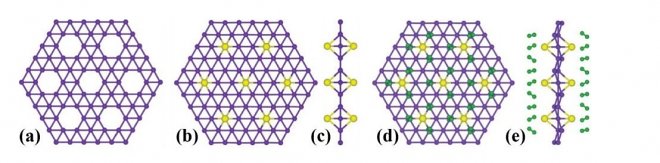
Borophene is synthesized by the deposition of boron vapors on silver surfaces. The resulting hexagonal lattice is one atom thick. At the same time, some atoms form 4 or 5 bonds accounting for borophene’s «holey» structure that explains its properties.
For instance, vacant spaces can be used to add atoms of another substance to give the material new properties. Very lightweight and highly flexible, Borophene is much stronger than graphene. Scientists see ion batteries as its main application, because the material is an excellent superconductor, which makes it a perfect candidate for storing ions.
The material can also be used as a catalyst for hydrogen reactions, not to mention its great importance for a breakthrough in the energy sector.
Share this with your friends!




Be the first to comment
Please log in to comment